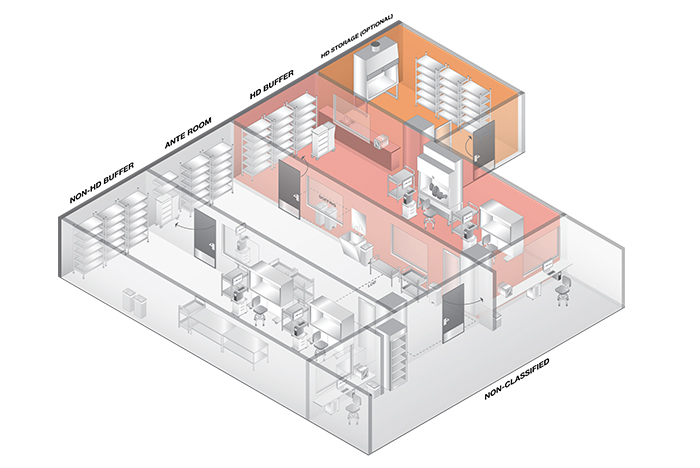
A compounding pharmacy with optional hazardous drug storage room.
Image courtesy of ASHE
United States Pharmacopeia (USP) General Chapter 800, Hazardous Drugs — Handling in Healthcare Settings, was developed to define the quality standards for the handling of hazardous drugs (HDs) and the proper environmental controls for compounding to protect health care workers and patients.
This exposure can occur with workers who are unaware of their exposure and in departments outside of the pharmacy. Other risks associated with HDs include compounding errors that pose additional risk of microbial contamination to patients.
Under the premise of protecting the health care worker and the patient, the chapter covers in detail requirements for all potential tasks where exposure can occur. Aspects of handling HDs covered in USP 800 include receiving, transporting, storing, compounding, dispensing, administering, spills, cleaning and waste disposal.
The American Society for Health Care Engineering (ASHE) has published a monograph titled, “Physical Environment Provisions of USP <800> ‘Hazardous Drugs — Handling in Healthcare Settings,’” which will be introduced during a presentation on the subject at this month’s 56th ASHE Annual Conference & Technical Exhibition in Baltimore, along with a compliance tool developed by the American Society for Health Care Risk Management. This article is excerpted from that monograph.
A look at HDs
A clear definition of HDs is critical so that health care workers can recognize the drugs they are handling, understand the risks and take proper actions to eliminate their exposure. The most commonly referenced HDs in many care settings are chemotherapy agents, as they are associated with cancer risk, but several other hazardous drugs that workers are exposed to can cause adverse health effects.
Resources
USP 800 utilizes the list of HDs identified by the National Institute for Occupational Safety and Health. Drugs are classified as hazardous if they possess characteristics such as genotoxicity, organ toxicity, teratogenicity or development toxicity, reproductive toxicity and carcinogenicity.
Health care providers are required to develop and keep a list of HDs utilized at their facility on file and available for surveyors.
Prior to the development of USP 800, the main guidance related to the handling of HDs was covered in USP General Chapter 797, Pharmaceutical Compounding — Sterile Preparations. In simplistic terms, the main intent of USP 797 was to protect hazardous and nonhazardous drugs from contamination.
Standards for preparing sterile drugs to reduce the risk for contamination, infection or incorrect dosing are defined throughout USP 797. What USP 797 did not cover was the handling of HDs and the associated risk of exposure of patients and health care workers. USP 800 was developed for this reason and to provide guidance on protecting any individual who may have exposure to HDs.
Planning and budgeting
As pharmacies look to modify their current operations to ensure compliance with USP 800, it is recommended traditional dollar per square foot values are not utilized for initial budgeting until the entire scope of construction is understood. Many factors can impact the overall renovation cost for compliance.
One of the biggest challenges for a pharmacy renovation is keeping the existing pharmacy operational while the spaces are being renovated. Some locations have the luxury of soft space that allows for smoother construction with the establishment of a temporary pharmacy that can be utilized during construction, but this is not always the case. It is important that construction phasing be evaluated during the planning process and that the quantity of phases be balanced between limiting staff disruption and construction cost.
Another item that can greatly impact construction cost and schedule is the condition and capacity of the existing HVAC system. The airflow, air quality, pressurization, temperature and humidity requirements in USP 800 make it risky to assume the existing infrastructure is adequate to comply with the guideline. Therefore, premeasurement and verification of the HVAC systems is highly recommended to identify all deficiencies prior to establishing a construction budget. The cost for addressing the deficiencies can be substantial depending on their magnitude.
In instances where the cost of compliance is either too disruptive to existing operations or not cost effective, some health systems have taken a hard look to determine if their compounding needs are better served at a different location. Many systems have decided to move their compounding needs to an off-site location such as a medical office building, which is a less expensive occupancy.
Physical considerations
The common spaces found in a working pharmacy include the general pharmacy, anteroom and buffer rooms; sometimes a storage room or HD storage room are included. Before understanding when these spaces are required and how they interact with one another, it is important to understand a few basic definitions and terminology utilized in USP 800. They include:
International Organization for Standardization (ISO) classification. Clean rooms are classified based upon the cleanliness of the air within the space. The lower the ISO classification number, the cleaner the air quality; ISO 1 is the cleanest, and ISO 9 is the least clean.
Direct compounding area (DCA). The DCA is the critical area within the ISO Class 5 hood where the compound is being prepared.
ISO 5. The containment primary engineering control (C-PEC) or hood where the compounds are mixed.
ISO 7. Buffer area where C-PEC is located.
ISO 8 or ISO 7. The classification of the anteroom directly adjacent to the buffer room.
Beyond use date (BUD). BUD is the time after which a compounded preparation cannot be used or stored. Compounded preparations that have a 12-hour or less BUD have less restrictive requirements for the classification of the room where the compounding occurs. This is described in the containment segregated compounding area (C-SCA) section.
C-PEC. C-PEC is the device, commonly referred to as the hood, where compounds are mixed. The C-PEC includes containment ventilated enclosures known as powder hoods, biological safety cabinet and compounding aseptic containment isolators. The National Sanitation Foundation classifies safety cabinets to differentiate their containment capabilities and performance levels. Compounding pharmacies in a health care application utilize a Class II and either a Type A2 or B2 for the C-PECs.
Containment secondary engineering control (C-SEC). C-SEC is the room where the C-PEC device is located. The C-SEC may be an ISO 7 buffer room with an ISO 7 anteroom or an unclassified C-SCA. The rooms are often referred to as the “positive” room or the “negative” room, but the terminology utilized in USP 800 is the buffer room and, more specifically, the non-HD buffer room and HD buffer room.
Compounding arrangements
The most common and recommended compounding arrangement defined in USP 800 is where HD buffer and non-HD buffer share an anteroom. In this configuration (below), staff enters the clean room by entering the anteroom. From this location — after donning PPE — staff can enter either compounding room. The anteroom often has windows, allowing staff to see into both compounding rooms to see if they are occupied prior to entering.
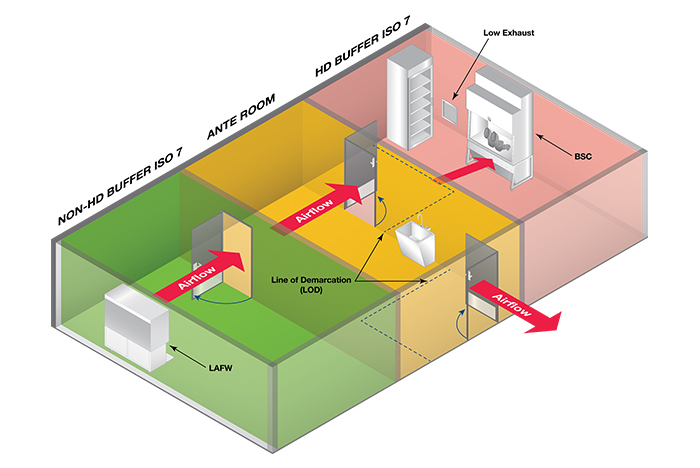
Image courtesy of ASHE
The second compounding arrangement discussed in USP 800 — though noted as not recommended, but allowed — is where the HD buffer room is entered through the non-HD buffer room. Operationally, this configuration (seen below) presents many challenges to pharmacy staff. Precautions must be made when transporting HDs and HD waste through the non-HD room to minimize the risk of cross-contamination. This is achieved through sealed containers and carts or the use of “pass-throughs” from the HD buffer room to an adjacent space.
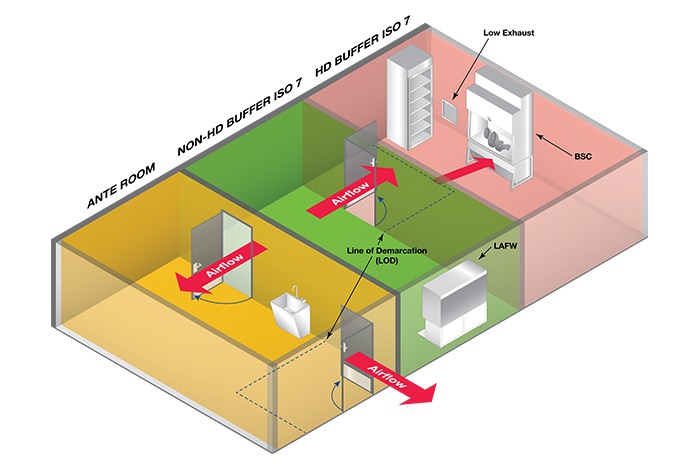
Image courtesy of ASHE
Beside the potential risk for cross-contamination, this arrangement is disruptive to staff in the non-HD buffer room every time staff passes through to the HD buffer room. This arrangement may be useful in an existing condition where a pharmacy is planning a renovation. If their current configuration or other space restrictions do not allow them the preferred arrangement, this arrangement may be useful to help minimize construction phasing and disruption to operations.
Hazardous drug storage
One of the biggest impacts USP 800 has on many current operations is the requirement related to HD storage. To prevent cross-contamination and potential staff exposure, USP 800 does not allow non-HDs and HDs to be stored within the same room. HDs must be stored in a negative pressure room with a minimum of 12 air changes per hour (ACH). Prior to USP 800, it was common to have HDs and non-HDs stored in refrigerators in the anteroom. This is no longer allowed.
Options to address this change are either storing the HDs within the HD buffer room or providing a dedicated HD storage room. Often the HD storage room is preferred because it allows for a location to unbox and store the HD, but this does require the planning of an additional room.
A key item related to HD storage that is often misunderstood and is found in section 5.2 of USP 800 states: “Sterile and nonsterile HDs may be stored together, but HDs used for nonsterile compounding should not be stored in areas designated for sterile compounding to minimize traffic into the sterile compounding area.”
Under this requirement, if a pharmacy will be conducting both nonsterile and sterile HD compounding, it will need a dedicated HD storage room. Options include:
- Store HDs in an HD buffer room (or C-SCA if applicable)
- A dedicated HD storage room
Under either HD storage room option, USP 800 requires all refrigerated antineoplastic HDs to be stored in a dedicated refrigerator. USP 800 states that if a refrigerator is located in the HD buffer room, an exhaust located adjacent to the refrigerator’s compressor and behind the refrigerator should be considered. The intent of this recommendation is to place a low wall exhaust to help draw out any particulates from the fridge or spillage from its contents. Though not clearly stated in USP 800, it is best practice to have this low wall exhaust behind the refrigerator for both a dedicated HD storage room and when HDs are stored in the HD buffer room.
Pass-throughs
Pass-throughs are enclosures with interlocking doors that are utilized in clean room spaces. They are common between the anteroom and buffer rooms or between the HD storage room and HD buffer room. Pass-throughs are discussed in both USP 797 and UPS 800. Important items include:
- Pass-throughs serving negative pressure rooms need to have sealed doors.
- Pass-throughs serving a storage room, such as the HD storage room that may be fire rated, need to be a rated assembly.
- Refrigerated pass-throughs are no longer allowed into the HD buffer room.
HVAC considerations
The intent of the HVAC design for the compounding areas — which consist of the HD buffer, anteroom and non-HD buffer — should be to meet clean room fundamentals. In basic terms, this means to provide a large amount of high-efficiency particulate air (HEPA)-filtered supply air at a low velocity at the ceiling and then remove the air with low return grilles to sweep out any particulates in the space.
The ISO 7 and ISO 8 rooms will require a laminar airflow diffuser (LAF) with HEPA filters at the ceiling to provide clean and low-velocity air. LAF provides unidirectional airflow that does not introduce turbulence and provides additional protection from bacterial shedding associated with personnel or surfaces in the space. Low wall returns are required for all the compounding areas to draw the air downward.
One challenge that USP 800 has introduced is defining a negative pressure range for the HD buffer room and the C-SCA. It is important that the HVAC design be flexible and resilient to meet these requirements. Construction quality can have a big impact on the relative tightness of the room, so this should be considered when the HVAC systems are selected.
Other HVAC items to consider include:
- Temperature and humidity must be monitored and documented for every day compounding occurs.
- Compounding rooms must be equipped with a pressure-monitoring system to notify occupants if spaces are within tolerance.
Other considerations
Several items are not specifically called out in 800 or 797 but should be considered during the planning stages. They include:
Pressure ports. As part of the six-month certification process, the pharmacy certifier will be testing the pressure drop across the HEPA-filtered diffusers to determine effectiveness. It is recommended as part of the design, tubing be run from the HEPA-filtered diffusers to a common test port located in the ceiling of the general pharmacy or non-classified space. This allows testing of the HEPA filters be done without disruption to the compounding areas.
Cameras. Cameras for security and monitoring are common requests within the general pharmacy and compounding areas. Oftentimes the compounding hoods are provided with internal cameras for monitoring of preparations. Any camera that is selected must be able to stand up to routine cleaning.
Music. There is a debate within the pharmacy industry on whether music should be allowed to be played in the compounding rooms. Some believe staff should be focusing on their preparations rather than music playing in the room, while others feel music improves alertness. If music is allowed by the pharmacy department, it is important staff do not bring in their personal music devices. This will be a source for outside contaminants and a potential particle generator. Many pharmacies are utilizing smart speakers that remain in the compounding room. They can be voice controlled by the occupants, operating hands free. Whichever device is selected, it should be able to stand up to routine cleaning.
Compounding room doors. Hands-free swinging or sliding doors are acceptable in the compounding area. When utilizing swinging doors, the direction of door swing is not defined in either 797 or 800, but it is recommended consideration be made for the movement of the doors and its impact on airflow. Some pharmacies have utilized interlocks that only allow one door to be open at a time to minimize impact on airflow and pressurization. Other items to consider are the door swing direction’s impact on staff flow and usable space. Whether a swinging or sliding door is utilized, it is important to review the needs of the occupants and factor in the impact on space and airflow.
ACH for ISO 7. For ISO 7 spaces, the minimum ACH is 30. A system that is provided with just the minimum ACH at startup may not be able to provide that minimum over the life of the system. It is recommended designers factor in filter loading and duct leakage when sizing the HVAC systems. Depending on system type and the size of the area served, designers may consider designing the space above the minimum 30 ACH to ensure compliance over a reasonable time period.
Defining the standards
USP 800 was developed to define the standards for the handling of HDs and the proper environmental controls to protect health care workers and patients. It is intended to be utilized with USP 797 to improve safety and quality for all those impacted by the pharmacy operations.
When discussing compliance needs, it is important to include individuals from not only the pharmacy department, but also facilities, administration, design and construction to ensure all needs are represented and understood.
Mike Zorich, PE, LEED AP, principal and director of health care at IMEG Corp., Chicago, authored the ASHE monograph, “Physical Environment Provisions of USP <800> ‘Hazardous Drugs — Handling in Healthcare Settings,’” from which this article was excerpted. His contributors were Chad Beebe, AIA, FASHE, CHFM, ASHE’s deputy executive director for advocacy, and Mike Lawless, LEED AP, a principal at IMEG Corp.