|
|---|
| This initiative is funded by an unrestricted educational grant from Clorox Healthcare™. |
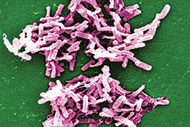
A growing push is on to better educate hospital leaders in preventing Clostridium difficile infection (CDI). And while much of the focus has been aimed at getting hospitals to implement more effective antibiotic stewardship programs, there has been an increased emphasis on the role the environment can play in harboring and serving as a potential transmission source for C. difficile spores.
In June, the Society for Healthcare Epidemiology of America (SHEA) in collaboration with the Infectious Diseases Society of America (IDSA), the American Hospital Association, the Association for Professionals in Infection Control and Epidemiology (APIC), the Joint Commission and others published updated practice recommendations on strategies to prevent CDI in acute care hospitals. A large section of the guidance published in the June issue of Infection Control & Hospital Epidemiology (ICHE) is devoted to following protocols that will ensure proper cleaning and disinfection of medical equipment and the patient care environment.
“Thorough environmental cleaning should be a core component of any effective program to prevent C. difficile infections in hospitals,” says Erik R. Dubberke, M.D., MSPH, associate professor of medicine at Washington University School of Medicine in St. Louis and medical director of infection control at Missouri Baptist Medical Center, who co-authored the SHEA/IDSA guidance paper.
Consistently achieving this fundamental goal is paramount at a time when CDI continues to exact a high price in patient mortality and costs to hospitals. Several studies have shown that C. difficile now surpasses methicillin-resistant Staphylococcus aureus (MRSA) as the most common organism to cause health care-associated infections in the United States, the SHEA/IDSA practice recommendation notes.
Mortality attributed to CDI is estimated to be 5 to 10 percent, various studies have shown, leading to an estimated 14,000 to 20,000 deaths in this country each year. In addition, epidemiologists report that CDI cases have been increasing in severity. Research by Dubberke and Margaret A. Olsen published in Clinical Infectious Diseases in 2012 noted that analysis of the best available data from 2008 indicated that CDI may have resulted in $4.8 billion in excess cost to U.S. acute care facilities. And despite indications that CDI rates nationally may have peaked, CDI remains at historically high levels.
All of this makes it essential for hospitals and environmental services (ES) teams to continuously improve cleaning, disinfection and hand-hygiene practices — from the purchase of EPA-registered disinfectants through ensuring proper application of these products according to manufacturer labels, and monitoring and reporting on hand-hygiene compliance and thoroughness of cleaning environmental surfaces.
Because C. difficile bacteria have a hard shell that enables these organisms to survive for months on such patient room surfaces as tables, floors, bedrails and toilets, proper cleaning processes must continue to be emphasized to ES associates of all experience levels, Dubberke explains.
“The most important thing in terms of education is that C. difficile bacteria contain spores, which is not an issue with other hospital bacteria of concern,” Dubberke says. Unless thorough cleaning with sufficient friction or “elbow grease” and proper disinfectants are applied for the correct amount of time, C. difficile spores can remain on surfaces and be transferred easily from infected patients to caregivers and, ultimately, to other patients.
In a commentary published in the July issue of ICHE, William A. Rutala, Ph.D., MPH, and David J. Weber, M.D., MPH, underscored that when evaluating disinfectants it’s important to recognize that some pathogens, such as C. difficile, are intrinsically more resistant to disinfectants. With this in mind, it’s important to pay close attention to disinfectant labels for organism kill times and surface contact or dwell time.
“The amount of disinfectant left on the surface is important, as it affects the contact time and the concentration of active ingredients delivered to the surface,” Rutala and Weber explain in the commentary. They add that using an inadequate amount of liquid per surface or “damp dusting” with a barely wet cotton cloth or disposable disinfectant wipe will not result in the desired antimicrobial reduction.
“Similarly, results have demonstrated efficient transfer of C. difficile spores from contaminated to clean surfaces by nonsporicidal wipes and overused sporicidal wipes. In contrast, wiping with sporicidal agents eliminated 3.90 log(10) C. difficile spores by inactivation and/or physical removal,” the authors reported.
In its “Guide to Preventing Clostridium difficile Infections” published in 2013, APIC notes that even though many EPA-registered germicides kill vegetative C. difficile, only chlorine-based disinfectants and high-concentration hydrogen peroxide formulations kill spores. The guide also notes that several sporicidal agents containing chlorine or hydrogen peroxide formulations are acceptable for use as general surface disinfectants.
The updated SHEA/IDSA practice recommendation calls for performing decontamination of rooms of patients with CDI using sodium hypochlorite diluted 1:10 with water or an EPA-approved sporicidal product in an outbreak or hyperendemic setting.
In addition to these steps for processing rooms where C. difficile patients were treated, it’s important to monitor, measure and report on the thoroughness of cleaning high-touch surfaces. This can be achieved with adenosine triphosphate testing or ultraviolet light gel marking systems. Dubberke believes it’s important for ES departments to be in charge of this process and to be accountable for the results.
“Ideally, this will be done on a somewhat real-time basis. The sooner you can provide feedback to the person doing the cleaning, the more effective that feedback will be,” Dubberke says.
Ultraviolet light disinfection systems and hydrogen peroxide vapor systems also have emerged as supplemental technologies to kill C. difficile and other bacteria. These technologies, however, should be used only after surfaces have been thoroughly cleaned.
Monitoring and measuring hand-hygiene compliance also remain essential tasks in the fight against CDI. While many hospitals historically have relied on visual observation, many technologies are available to aid in this effort. The SHEA/IDSA guidance emphasizes the need for “meticulous hand hygiene on the basis of Centers for Disease Control and Prevention or World Health Organization guidelines before and after entering the room of a patient with CDI.” The guidance also notes that washing hands with soap and water is preferred over alcohol-based, hand-hygiene products after caring for a patient with CDI in outbreak or hyperendemic settings.
Bob Kehoe is the associate publisher of Health Facilities Management.
Resources
- Strategies to Prevent Clostridium difficile Infections in Acute Care Hospitals: 2014 Update; SHEA/IDSA Practice recommendations
- EPA’s Registered Antimicrobial Products Effective Against Clostridium difficile Spores
- Guide to Preventing Clostridium difficile Infections; APIC Implementation Guide
- Clostridium difficile Prevention Tool Kit