What to expect during certification of compounding spaces
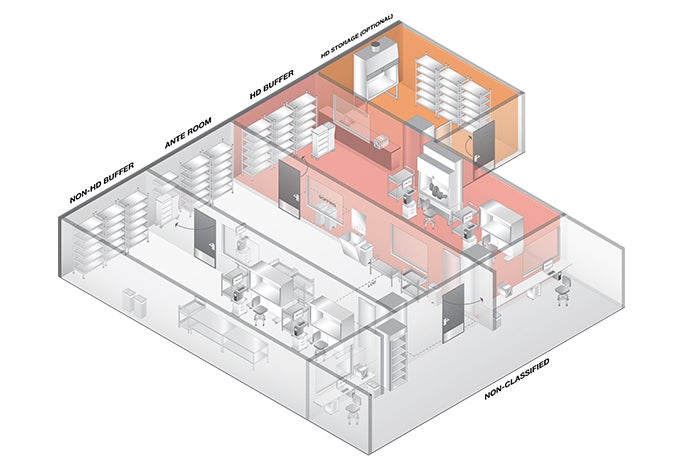
The requirement for certification of the air quality, airflow and pressurization in the compounding areas is still found in Section 4.6 of USP General Chapter 797, Pharmaceutical Compounding — Sterile Preparations, but the similar physical environment requirements of the hazardous drug compounding area defined in USP General Chapter 800, Hazardous Drugs — Handling in Healthcare Settings, will be reviewed in the certification process as well.
Certification is required at least every six months using procedures defined by the current Controlled Environment Testing Association certification guide for “Sterile Compounding Facilities.” The certification of the space includes the following:
- Airflow testing performed to determine proper air changes per hour and space pressurization.
- HEPA filter testing performed to determine integrity and condition of HEPA filters to determine performance is met and leakage is not occurring.
- Total particulate count testing performed under dynamic operating conditions, air and surface, to provide information on environmental quality of the spaces (determine both viable and nonviable particulates).
- Other certification tasks such as certification of containment primary engineering control and cytotoxic residue sampling.
The American Society for Health Care Engineering’s monograph, “Physical Environment Provisions of USP <800> ‘Hazardous Drugs — Handling in Healthcare Settings,’” includes more information on enforcement.