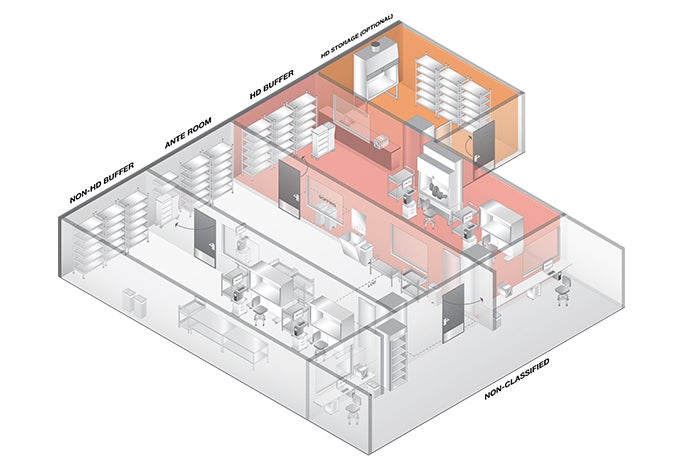
Plan review for compounding rooms
The pharmacy staff should be highly engaged by the design and construction team early in the planning stages for compliance with USP General Chapter 800, Hazardous Drugs — Handling in Healthcare Settings.
These discussions should start with understanding the entire process for how staff receives, transfers, stores, delivers and administers hazardous drugs (HDs) to patients. These discussions also should include the techniques the pharmacy staff will utilize for HD compounding.
It’s critical the designers understand how soon medications will be administered following compounding, and if the pharmacy staff will be performing nonsterile compounding, sterile compounding or both. Knowing these differences will allow for planning a compliance solution that meets the pharmacy’s specific needs.
The American Society for Health Care Engineering’s monograph, “Physical Environment Provisions of USP <800> ‘Hazardous Drugs — Handling in Healthcare Settings,’” includes a suggested checklist for use during design plan and review.