Design distinctions for exam, procedure and operating rooms
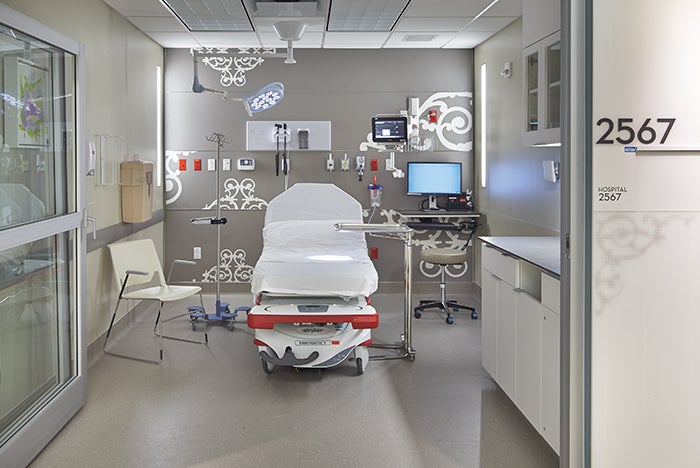
A procedure room is a more restrictive environment than an exam room and less restrictive than an OR.
Image © Benjamin Benschneider (courtesy of NBBJ)
A question frequently asked by designers working on health care projects is, “Which procedures can be done in an examination, procedure and operating room (OR)?”
To help clarify this issue, the 2018 Health Guidelines Revision Committee (HGRC), the body responsible for development of the 2018 edition of the Facility Guidelines Institute’s (FGI’s) Guidelines for Design and Construction, worked to align the definitions and requirements for these basic room types.
An understanding of appropriate uses for these rooms can lead to spaces designed and constructed for their intended use, reducing costly over-building of unneeded infrastructure and eliminating under-building of spaces that do not support their intended use.
Recognizing the importance of this understanding should encourage facility owners, planners and designers to initiate conversations with their constituents, clients and users early in the project planning process.
Procedure types
It is commonly understood in the health care field that the appropriate environment for diagnostic, treatment and noninvasive procedures is an exam or treatment room and that invasive procedures are to be performed in an OR.
There is less clarity when it comes to identifying the types of procedures for which a procedure room is the appropriate setting. This confusion stems from the fact that the level of invasiveness of medical procedures falls along a spectrum between noninvasive and invasive. The term “minimally invasive” has sometimes been used; however, this is a term eschewed because of its innate contradictions. Steps to identify where a procedure falls on the invasiveness spectrum include:
Perform a clinical risk assessment. Determining the appropriate environment for procedures that fall between noninvasive and invasive begins with an understanding of the level of invasiveness of the intended procedure and the perceived level of risk to the patient. These distinctions must be made early in the planning process and require input from a multidisciplinary team that works together to develop a clinical risk assessment. A robust multidisciplinary team includes representatives from the surgery, anesthesiology, infection prevention, facility management and design teams, as well as the facility regulatory officer.
An in-person meeting of all parties participating in the clinical risk assessment is recommended. If one or more of the parties cannot attend a scheduled meeting, it is preferable to reschedule. If one party is missing, an attendee should be made responsible for follow-up with that person with a timeline to report back to the group prior to issuance of final meeting minutes.
The clinical risk assessment developed by this team can be used to determine the appropriate environment for current and future procedures to be performed in the project under development.
Classify the planned procedures. The multidisciplinary team performing the clinical risk assessment for a new project must collaborate to discuss and determine which procedures will take place in the room, how the room will be used, what level of invasiveness is to be planned for, what acuity and type of patient are expected, and whether and what type of anesthesia will be introduced.
These factors are looked at in conjunction with more practical concerns such as whether this is a renovation or new construction, available floor-to-floor height, budget and cost, infrastructure needs, access and location of the room, preoperative and recovery patient care needs, and desired future flexibility of the space to be built.
In addition to addressing planned procedures, the clinical risk assessment must consider the likelihood that other procedures — possibly with different needs — may be performed in the space in the future. To provide the greatest flexibility for the future requires designing to a more restrictive standard, which is not always practical or possible. Early and open dialogue with all parties involved with a project and its planned uses is necessary to manage expectations.
The first step in classifying the procedures to be performed in a new space, whether new construction or renovation, is to understand the definition of an invasive procedure. Following is the 2018 Guidelines definition of the term, with changes from the 2014 edition in bold:
“Invasive procedure: A procedure that is performed in an aseptic surgical field and penetrates the protective surfaces of a patient’s body (e.g., subcutaneous tissue, mucous membranes, cornea). An invasive procedure may fall into one or more of the following categories:
- Requires entry into or opening a sterile body cavity (i.e., cranium, chest, abdomen, pelvis, joint spaces)
- Involves insertion of an indwelling foreign body
- Includes excision and grafting of burns that cover more than 20 percent of total body area
- Does not begin as an open procedure but has a recognized measurable risk of requiring conversion to an open procedure”
Invasive procedures must be performed in the cleanest environment — an OR — because they typically expose usually closed parts of a patient’s body to potential invasion by pathogens (i.e., infection-causing agents), thereby increasing infection risk. Other procedures that breach the body’s surfaces without opening them up to the surrounding environment may be safely performed in a procedure room.
Another factor to consider in classifying the procedures is anesthetic use. One myth that needs to be dispelled is that the use of inhalation or general/block anesthetic means a procedure is invasive and must be performed in an OR setting. Inhalation anesthetic does not an OR make. Although inhalation anesthetic is often required for invasive procedures, it also may be used for diagnostic services and noninvasive procedures because of the type of patient rather than the type of procedure. For instance, a patient may need an inhalation anesthetic to overcome anxiety or to remain still throughout a procedure.
Exam or procedure rooms for which use of inhalation anesthetic is planned must follow the requirements of the National Fire Protection Association’s NFPA 99, Health Care Facilities Code, and ANSI/ASHRAE/ASHE Standard 170, Ventilation of Health Care Facilities. (There are some discrepancies between Standard 170 and the Guidelines; however, ASHRAE and FGI are working to align the documents.)
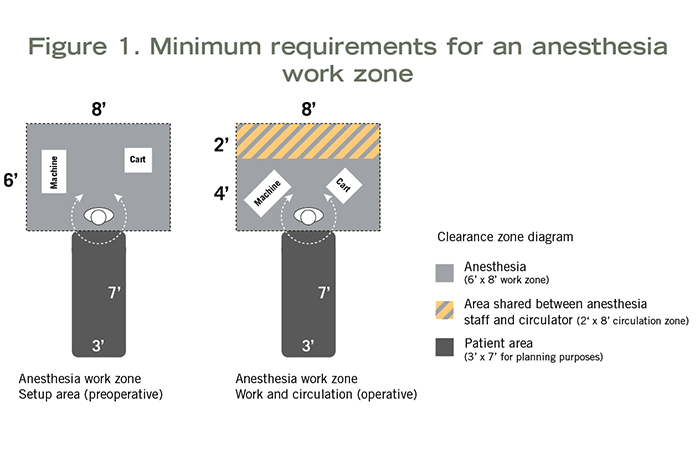
When inhalation anesthetic will be used in an exam room, procedure room or OR, space to accommodate use of that equipment must be planned into the design. The 2018 HGRC established minimum planning requirements for an anesthesia work zone. This was based on the work of a multidisciplinary team of the HGRC, which included an anesthesiologist, a surgeon, an OR nurse and an architect. This team explored the minimum space needed to safely set up anesthesia equipment and administer inhalation anesthetic during a procedure, including simulation of a surgery case in a real OR.
Measurements taken during the simulation (see Figure 1) indicated the following spaces were needed:
- 8 feet wide by 6 feet deep (48 square feet) at the head of the patient to safely set up anesthesia equipment pre-procedure.
- 8 feet wide by 4 feet deep (32 square feet) at the head of the patient to administer anesthesia during the procedure.
Distinctions between rooms
Each of the three basic clinical spaces — examination rooms, procedure rooms and ORs — has its own set of requirements in the FGI Guidelines and criteria for design considerations, infrastructure and operations. A closer examination of the differences includes:
Exam room. The simplest of the three basic room types is the examination (or treatment) room. [In this article, the term “exam room” will be used to indicate both exam and treatment rooms. In the Guidelines, the term “treatment room” is used only when referring to rooms in an emergency or urgent care facility.]
The exam room is an unrestricted area that is accessed from an unrestricted area. It is used for patient consultation, examination, and various noninvasive treatments and procedures. There is an expectation of physical contact, or “laying on of hands,” between the caregiver and patient.
A general description of the patient care permitted in an exam room is treatments or procedures that may require high-level disinfected or sterile instruments but do not require the environmental controls of a procedure room. Some examples include blood draws, injections/shots, minor cuts and sprains (including wound packing), stitches and casting, minor dermatological procedures (including removal of skin tags), PICC (percutaneously inserted central catheter) line placement and removal, and needle biopsies.
The exam room is the simplest in terms of air changes per hour — typically two outside air changes per hour, and four or six total air changes per hour (depending on the use of the room; see ASHRAE 170 for details). There are requirements for a standard diffuser and return array but no pressure relationship to adjacent areas. Finishes are cleanable and washable.
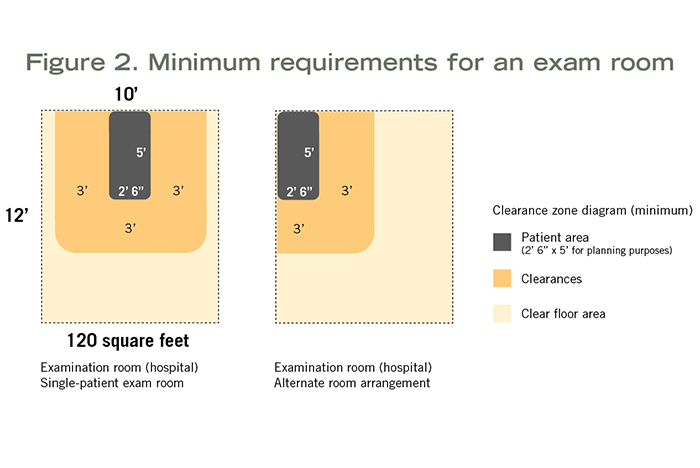
Minimum clear floor area and room dimensions vary depending on whether it is a hospital-based exam (120 square feet, 10 feet wide), outpatient-based exam (80 square feet) or outpatient-based specialty exam (100 square feet) room. Each of these has specific clearance requirements around the exam table, gurney or chair. Clearances and possible furniture arrangements are shown in Figure 2.
Procedure room. The procedure room is more restrictive than the exam room and less restrictive than the OR. The procedure room is a semi-restricted area and, whereas the exam room is accessed from an unrestricted area, the procedure room can be accessed from either an unrestricted or semi-restricted area. This access is one of the operational and planning decisions that must be discussed by the multidisciplinary clinical risk assessment team as it has operational implications (e.g., staff attire, cleaning and infection prevention).
The procedure room is defined as a room “designated for the performance of patient care that requires high-level disinfected or sterile instruments and some environmental controls but is not required to be performed with the environmental controls of an OR.” The room is intended for procedures in which the body cavity or protective surface of the body may be penetrated but which do not meet the definition of “invasive.” Examples include implantation of IV ports and placement of temporary foreign bodies (e.g., Hickman catheters and pacing wires).
The typical procedure room requires three outside air changes and 15 total air changes and a standard diffuser and return array. The diffusers are required to be above the patient area and Group E or A non-aspirating type. A positive pressure relationship to adjacent areas also is required. Finishes must be scrubbable and free of crevices and fissures.
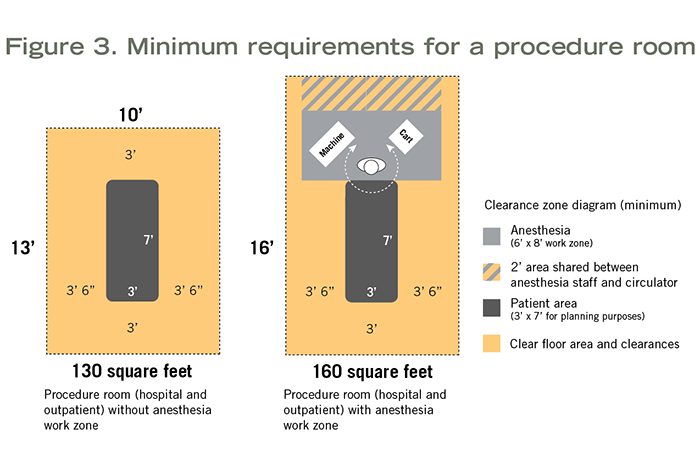
Minimum clear floor area and room requirements are the same whether the procedure room is hospital-based or outpatient-based. These rooms can be planned for procedures that do not require inhalation anesthetic (130 square feet, 10 feet wide) or for procedures that do (160 square feet, which includes the anesthesia work zone; 10 feet wide). For more details, see Figure 3.
Some procedure rooms are designed for specialized purposes and have their own specific requirements, such as rooms for bronchoscopy (negative pressure relationship) and endoscopy (clear floor area) procedures.
Operating room. The OR has the most restrictive and robust minimum infrastructure requirements of the basic room types and is a restricted area that can only be accessed from a semi-restricted area.
An OR is defined as a room “that meets the requirements of a restricted area, is designated and equipped for performing surgical or other invasive procedures, and has the environmental controls for an OR as indicated in ASHRAE 170.” An aseptic field is required for all procedures, which results in the requirement for the unidirectional diffuser array.
Procedures in this room typically meet the definition of “invasive procedure” and need to be performed in the cleanest environment. Examples of invasive procedures performed in an OR include joint replacement surgery, open heart surgery, mastectomy, hysterectomy, appendectomy, cataract surgery, burn excision and arthroscopy.
An OR requires a minimum of four outside air changes per hour and 20 total air changes per hour, a unidirectional diffuser array and low sidewall returns, and a positive relationship to adjacent areas. Finishes are monolithic, scrubbable, and free of crevices and fissures. Minimum clear floor area and clearances are different for hospital-based and outpatient-based ORs.
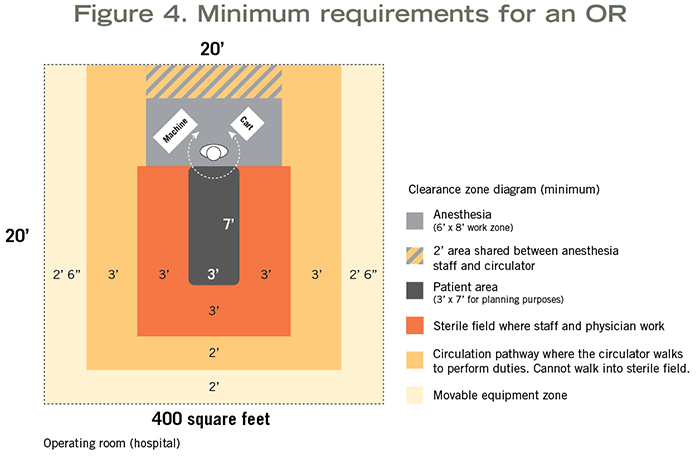
Hospital-based ORs assume inhalation anesthetic will be used and require a minimum clear floor area of 400 square feet with a 20-foot minimum clear dimension (see Figure 4). If specialty procedures are to be performed (e.g., neuro, ortho or cardiac) requiring additional equipment and staff, the room must have a minimum clear floor area of 600 square feet.
Outpatient-based ORs can be smaller. These rooms are typically planned for invasive procedures for which it is assumed inhalation anesthetic will be used (270 square feet, 15 feet wide); however, it is possible to plan outpatient-based ORs for invasive procedures that do not require inhalation anesthetic (225 square feet, 15 feet wide). Before exercising this option, however, whether this decision may limit future uses of the room should be carefully considered.
The FGI Guidelines provides minimum requirements, which, if not met, may compromise safety and operational efficiencies. The Guidelines does not recommend designing any room to the minimum requirements outlined in its pages. While the minimum requirements for an outpatient-based OR are significantly less than those for a hospital-based OR, it is common to see newly constructed outpatient-based ORs with a clear floor area of 400 to 650 square feet.
Appropriate uses
It is important for health care facility management teams, planners and designers to take the time at the start of a project to involve the right people and develop a clinical risk assessment based on the intended procedures, level of invasiveness, perceived risk of infection, acuity of the patients, amount of equipment, number of people needed and future flexibility expectations.
This will determine the type of room needed and will be the basis of design going forward.
Once a room with its infrastructure has been built, it becomes a limiting factor and will determine which procedures are safe to perform in it and which are not.
Thus, it is important for those responsible for health care project planning and design to have a general understanding of the differences among the three basic room types and the factors that determine when each is the appropriate setting for planned use.
About this article
This article is published by Health Facilities Management in collaboration with the Facility Guidelines Institute.
Bryan Langlands, AIA, ACHA, EDAC, is a principal with NBBJ. He can be reached at blanglands@nbbj.com.